
Main | Contact | Archives | Recipes
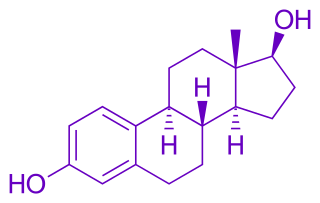
Estradiol
3.1. Synthesis of ent-17β-estradiol (i have no clue what any of this means)
Indenone 4 [33], the C,D-ring synthon, was treated with NaH in ethylene glycol dimethyl
ether and reacted with tosylate 5b [29] to afford compound 6 (60%).
Hydrogenation of
compound 6 gave a 52% yield of the indenones 7a (major isomer) and 7b (minor isomer).
Treatment of compound 7a with 10 N HCl at 0 °C results in epimerization of the
(methoxyphenyl)ethyl group to give 7b and subsequent cyclization leads to Δ9(11) ent-
steroid 8a (72%) and a small amount of the isomeric Δ8 ent-steroid 8b as products.
Under
these reaction conditions, compound 7b also yields products 8a and 8b.
The trans ring
fusion of the C,D-rings of ent-steroid 8a was established initially by 1H NMR and 13C NMR
spectroscopy.
The chemical shifts of the 18-Me group protons (δ = 0.78) and the carbon
resonance of this group (δ = 11.55) are both characteristic of the trans C,D-ring fusion of
ent-steroid 8a [34].
Hydrogenation of compound 8a produced ent-steroids 9a (81%, major
product) and 9b (minor product).
Removal of the tert-butyl protecting group from the
oxygen atom at C17 using 6 N HCl in THF/EtOH converts compound 9a to ent-steroid 10,
which was used without purification.
Removal of the methyl protecting group from the
oxygen atom at C3 using DIBALH [35] converts compound 10 to ent-17β-estradiol 3 (84%
yield overall for the 9a to 3 conversion).
Alternatively, methyl group removal from the
oxygen atom at C3 using 48% HBr [36] in glacial acetic acid gave a mixture of ent-steroids
3 (minor product) and 11 (major product).
The overall yield for the conversion of indenone
4 to ent-17β-estadiol is 15.2%
Stolen from This paper